What is RYTELO?
- with anemia (low red blood cell counts) who need blood transfusions of 4 or more red blood cell units over 8 weeks
and
- who have not responded to, have stopped responding to, or cannot be treated with medicines called erythropoiesis-stimulating agents (ESAs).
It is not known if RYTELO is safe and effective in children.
What is RYTELO?
RYTELO may work differently than other treatment options
RYTELO works on a part of the cell that affects an enzyme called telomerase, which is often more active in abnormal MDS cells
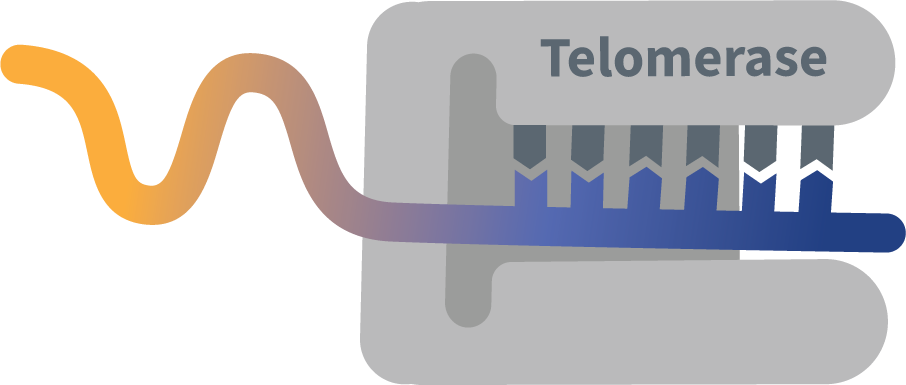
In nonclinical* studies, RYTELO helped to stop these abnormal MDS cells from multiplying
How was RYTELO (imetelstat) studied?
RYTELO was studied in the IMerge phase 3 clinical trial. 178 adult patients with low- or intermediate-1 risk MDS with anemia (low red blood cell counts) who need blood transfusions of 4 or more red blood cell units over 8 weeks, compared with no active medication (placebo), were studied across 17 different countries.
The goal of this trial was to see whether 118 patients treated with RYTELO would need fewer red blood cell transfusions compared with the 60 patients who received treatment with placebo.
Patients were included for the IMerge trial if their previous MDS treatment with an erythropoiesis-stimulating agent (ESA) did not work for them or had stopped working, or if they were ineligible for an ESA.
Patients could receive supportive care as needed, including red blood cell transfusions, platelet transfusions, and growth factors.

Not an actual patient.
What were the results from the clinical trial?

The primary trial objective was to determine the number of RYTELO patients who reached 8 consecutive weeks or more without a red blood cell transfusion.
Nearly 40% (n=47 out of 118) of patients taking RYTELO required
ZERO
red blood cell
transfusions for
8 consecutive
weeks or more
vs 15% (n=9 out of 60) of patients taking placebo
The secondary objective was to determine the number of RYTELO patients who reached 24 consecutive weeks or more without a red blood cell transfusion.
28% (n=33 out of 118) of patients taking RYTELO required ZERO red blood cell transfusions for 24 consecutive weeks or more vs 3% (n=2 out of 60) of patients taking placebo
What is RYTELO?
RYTELO (imetelstat) is a prescription medicine used to treat a condition called low- to intermediate-1 risk myelodysplastic syndromes (MDS) in adults:
- with anemia (low red blood cell counts) who need blood transfusions of 4 or more red blood cell units over 8 weeks
and
-
who have not responded to, have stopped responding to, or cannot be treated with medicines called erythropoiesis-stimulating agents (ESAs).
It is not known if RYTELO is safe and effective in children.
Important Safety Information
Before you receive RYTELO (imetelstat), tell your healthcare provider about all of your medical conditions, including if you:
-
are pregnant or plan to become pregnant. RYTELO may harm your unborn baby and may cause loss of pregnancy (miscarriage). Tell your healthcare provider right away if you become pregnant or think you may be pregnant during treatment with RYTELO.
Females who are able to become pregnant:
- Your healthcare provider will perform a pregnancy test before you are given RYTELO.
- You should use effective birth control (contraception) during treatment with RYTELO and for 1 week after your last dose.
- are breastfeeding or plan to breastfeed. It is not known if RYTELO passes into your breastmilk. Do not breastfeed during treatment with RYTELO and for 1 week after your last dose.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
What is the most important information I should know about RYTELO?
RYTELO may cause serious side effects, including:
-
Low platelet counts (thrombocytopenia). Low platelet counts are common during treatment with RYTELO and can also be severe. Low platelet counts can increase your risk for bleeding. Your healthcare provider may give you platelet transfusions to reduce the risk of bleeding if you develop a low platelet count during treatment with RYTELO. Tell your healthcare provider right away if you develop any signs or symptoms of bleeding, including:
- unusual bleeding or bruising
- bleeding that lasts a long time
- nosebleeds
- vomiting blood
- blood in your stool or black tarry stool
-
Low neutrophil counts (neutropenia). Low counts of a type of white blood cell called neutrophils are common during treatment with RYTELO and can also be severe. Low neutrophil counts can increase your risk for infections, including serious infections and sepsis. Your healthcare provider may give you medicines before you start treatment to help prevent neutropenia and infections and may treat you with medicines if you develop these problems during treatment with RYTELO. Tell your healthcare provider right away if you develop any signs or symptoms of infection during treatment with RYTELO, including:
- fever
- shortness of breath or trouble breathing
- cough
- chills
- pain or burning when you urinate
Your healthcare provider will do blood tests to check your platelet and neutrophil counts before starting treatment with RYTELO, weekly for the first 2 cycles of treatment, before you receive each additional cycle, and as needed during your treatment.
Your healthcare provider may delay your next treatment, decrease your dose, or stop treatment with RYTELO if you develop thrombocytopenia or neutropenia during treatment.
What are the possible side effects of RYTELO?
RYTELO may cause serious side effects, including:
- See “What is the most important information I should know about RYTELO?”
-
Infusion-related reactions. RYTELO can cause infusion-related reactions during or after your infusion that can be severe, including a severe sudden increase in blood pressure called hypertensive crisis. Your healthcare provider will give you medicines before each RYTELO infusion to help prevent or lessen infusion-related reactions and will watch you for at least 1 hour after your infusion. If you develop infusion-related reactions, your healthcare provider may infuse RYTELO more slowly, temporarily stop, or permanently stop your treatment. Tell your healthcare provider if you develop any signs or symptoms of infusion-related reactions, including:
- stomach pain
- joint pain
- weakness and tiredness
- back and bone pain
- diarrhea
- redness
- headache
- high blood pressure
- not feeling well
- chest pain that is not related to your heart
- itching
- hives
The most common side effects of RYTELO include:
- decreased platelet counts
- decreased white blood cell counts
- decreased neutrophil counts
- increased liver enzymes (AST, alkaline phosphatase, and ALT)
- tiredness
- longer than usual blood clotting times
- joint, bone and muscle pain
- Covid-19 infections
- headache
These are not all of the possible side effects of RYTELO. Call your doctor for more information and medical advice about side effects. You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/MedWatch or call 1-800-FDA-1088.
Please see RYTELO (imetelstat) full Prescribing Information, including Medication Guide.
Important Safety Information
Before you receive RYTELO (imetelstat), tell your healthcare provider about all of your medical conditions, including if you:
-
are pregnant or plan to become pregnant. RYTELO may harm your unborn baby and may cause loss of pregnancy (miscarriage). Tell your healthcare provider right away if you become pregnant or think you may be pregnant during treatment with RYTELO.
Females who are able to become pregnant:
- Your healthcare provider will perform a pregnancy test before you are given RYTELO.
- You should use effective birth control (contraception) during treatment with RYTELO and for 1 week after your last dose.
- are breastfeeding or plan to breastfeed. It is not known if RYTELO passes into your breastmilk. Do not breastfeed during treatment with RYTELO and for 1 week after your last dose.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
What is the most important information I should know about RYTELO?
RYTELO may cause serious side effects, including:
-
Low platelet counts (thrombocytopenia). Low platelet counts are common during treatment with RYTELO and can also be severe. Low platelet counts can increase your risk for bleeding. Your healthcare provider may give you platelet transfusions to reduce the risk of bleeding if you develop a low platelet count during treatment with RYTELO. Tell your healthcare provider right away if you develop any signs or symptoms of bleeding, including:
- unusual bleeding or bruising
- bleeding that lasts a long time
- nosebleeds
- vomiting blood
- blood in your stool or black tarry stool
-
Low neutrophil counts (neutropenia). Low counts of a type of white blood cell called neutrophils are common during treatment with RYTELO and can also be severe. Low neutrophil counts can increase your risk for infections, including serious infections and sepsis. Your healthcare provider may give you medicines before you start treatment to help prevent neutropenia and infections and may treat you with medicines if you develop these problems during treatment with RYTELO. Tell your healthcare provider right away if you develop any signs or symptoms of infection during treatment with RYTELO, including:
- fever
- shortness of breath or trouble breathing
- cough
- chills
- pain or burning when you urinate
Your healthcare provider will do blood tests to check your platelet and neutrophil counts before starting treatment with RYTELO, weekly for the first 2 cycles of treatment, before you receive each additional cycle, and as needed during your treatment.
Your healthcare provider may delay your next treatment, decrease your dose, or stop treatment with RYTELO if you develop thrombocytopenia or neutropenia during treatment.
What are the possible side effects of RYTELO?
RYTELO may cause serious side effects, including:
- See “What is the most important information I should know about RYTELO?”
-
Infusion-related reactions. RYTELO can cause infusion-related reactions during or after your infusion that can be severe, including a severe sudden increase in blood pressure called hypertensive crisis. Your healthcare provider will give you medicines before each RYTELO infusion to help prevent or lessen infusion-related reactions and will watch you for at least 1 hour after your infusion. If you develop infusion-related reactions, your healthcare provider may infuse RYTELO more slowly, temporarily stop, or permanently stop your treatment. Tell your healthcare provider if you develop any signs or symptoms of infusion-related reactions, including:
- stomach pain
- joint pain
- weakness and tiredness
- back and bone pain
- diarrhea
- redness
- headache
- high blood pressure
- not feeling well
- chest pain that is not related to your heart
- itching
- hives
The most common side effects of RYTELO include:
- decreased platelet counts
- decreased white blood cell counts
- decreased neutrophil counts
- increased liver enzymes (AST, alkaline phosphatase, and ALT)
- tiredness
- longer than usual blood clotting times
- joint, bone and muscle pain
- Covid-19 infections
- headache
These are not all of the possible side effects of RYTELO. Call your doctor for more information and medical advice about side effects. You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/MedWatch or call 1-800-FDA-1088.
Please see RYTELO (imetelstat) full Prescribing Information, including Medication Guide.
What is RYTELO?
RYTELO (imetelstat) is a prescription medicine used to treat a condition called low- to intermediate-1 risk myelodysplastic syndromes (MDS) in adults:
- with anemia (low red blood cell counts) who need blood transfusions of 4 or more red blood cell units over 8 weeks
and
-
who have not responded to, have stopped responding to, or cannot be treated with medicines called erythropoiesis-stimulating agents (ESAs).
It is not known if RYTELO is safe and effective in children.
